First Patients enrolled in the C03 clinical study in the UK
“We see the TIES® Solution as a potential step change for ileostomy patients, providing them with a better quality of life,” said Prof. David Jayne, who performed the procedure at St. James in Leeds. He continued, “We are delighted to be participating in the TIES® C03 study, as part of the NIHR MedTech in Surgical Technologies, to undertake a rigorous evaluation of this exciting new technology. There is a large demand for the TIES® device from our patients, but we need to be sure that it is safe and effective. In TIES® C03, we will be able to monitor and track the performance of the TIES® Solution, which is absolutely crucial for us. The wellbeing and safety of our patients is our main objective.”
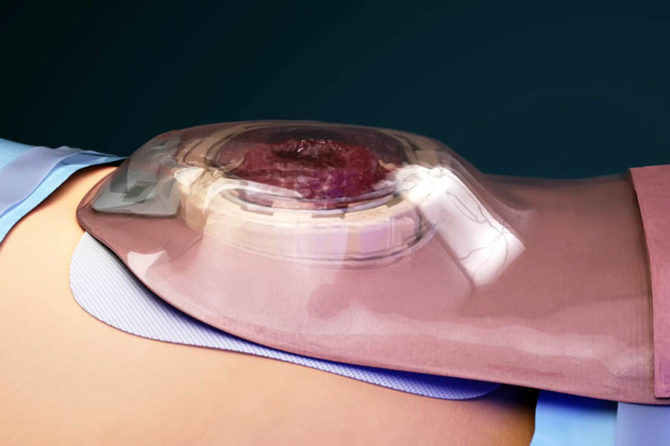
Patients who are candidates for the TIES® typically suffer from ulcerative colitis or other inherited intestinal diseases, but are often otherwise young and active. With the TIES® Solution, a previous Ileostomy can now be managed and controlled. About 22.000 patients annually receive an Ileostomy in Europe, and an estimated 260,000 live with ileostomies today. The TIES® procedure will also be available both for new and existing ileostomies.
“The TIES® Solution does not reverse an ileostomy, but it can give back the freedom to live an almost normal life with a chronic condition,” the CEO of OstomyCure AS, Dr Ben Broennimann states, and continues, “We are confident that the TIES® Solution works and that the continent solution it offers represents a tremendous improvement in patient quality of life. With the initiation of our multi-centre study of 200 patients, primarily in the UK and Sweden, we intend to scientifically prove our claim.”
“We also believe we will be able to quantify a significant health economic benefit for the TIES® Solution over the traditional bag systems,” continues Dr. Ben Broennimann. He adds, “About 60% of ileostomates today experience some level of often severe skin problems, along with leakage, unwanted odour and general discomfort. The TIES® Solution allows a tight stomal seal with no skin contact, which should significantly reduce these problems.”
About OstomyCure AS:
OstomyCure AS is a Medical Technology company headquartered in Oslo, Norway. The company develops a revolutionary technology; Transcutaneous Implant Evacuation System, The TIES® System.
The TIES® System is the result of several years of research combined with the latest 3D manufacturing technology, developed and produced in accordance with regulatory standards to ensure safety for patients.
The company is ISO 13485:2016 Annex II certified for its Quality Management System for Medical Devices. The TIES® System is CE marked since June 2016.
The market that OstomyCure addresses is estimated to be USD 2 billion per year.
The National Institute for Health Research (NIHR) is the nation’s largest funder of health and care research. The NIHR:
- Funds, supports and delivers high quality research that benefits the NHS, public health and social care
- Engages and involves patients, carers and the public in order to improve the reach, quality and impact of research
- Attracts, trains and supports the best researchers to tackle the complex health and care challenges of the future
- Invests in world-class infrastructure and a skilled delivery workforce to translate discoveries into improved treatments and services
- Partners with other public funders, charities and industry to maximise the value of research to patients and the economy
The NIHR was established in 2006 to improve the health and wealth of the nation through research and is funded by the Department of Health and Social Care. In addition to its national role, the NIHR supports applied health research for the direct and primary benefit of people in low- and middle-income countries, using UK aid from the UK government.
Contact information:
Dr. Johan Järte, M.D. CEO
OstomyCure AS
Email: jjaerte@ostomysecure.com
